Nov2022
CY6463 administration is linked to improvements in Alzheimer’s disease-relevant biomarkers, as revealed by eXplainable AI-driven analysis of multiple Phase 1 clinical trials


• Phase 1 studies show improvement in cognitive factors, consistent with therapeutic potential of CY6463 to improve key features in neurological diseases
• CY6463 favorable tolerability and safety profile was confirmed
Cyclerion Therapeutics’ CY6463 is a first-in-class, CNS-penetrant, soluble guanylate cyclase (sGC) stimulator that modulates a key node in a fundamental signaling pathway. CY6463 has been evaluated in two Phase 1 studies in healthy volunteers as well as signal-seeking patient studies in the rare mitochondrial disease, MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) and in CIAS (cognitive impairment associated with schizophrenia); a study in AD (Alzheimer’s disease) is ongoing. Cyclerion is advancing CY6463 for the treatment of rare mitochondrial diseases.
A total of 134 healthy participants were enrolled across two randomized, placebo-controlled, Phase 1 studies in which single-ascending doses, multiple-ascending doses, food effect (crossover design) and the pharmacology of CY6463 (crossover design) were evaluated. In each study, safety, pharmacokinetic, and endpoints assessments were collected at baseline and at the end of dosing. Previously reported favorable safety profile of CY6463 was confirmed, with no major adverse events.
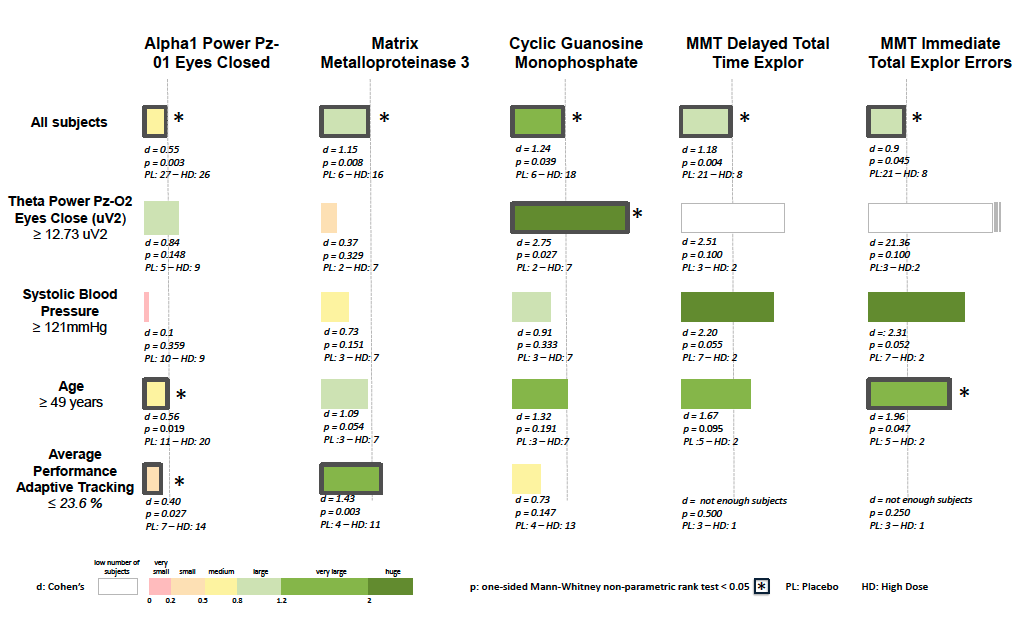
A systematic analysis of both trials using Ariana’s KEM® (Knowledge Extraction & Management) eXplainable AI platform revealed the impact of higher dose of CY6463 on 5 endpoints related to spatial learning (Milner Maze Test speed and accuracy, p=0.004 and p=0.045), brain activity (electroencephalography: increased alpha power, eyes closed, p = 0.003), inflammation (Matrix Metalloproteinase 3, p = 0.008) and target engagement (cyclic guanosine monophosphate, p=0.019), thus demonstrating the potential of CY6463 for the treatment of multiple neurological diseases.
Ariana’s KEM® also generated new hypotheses of patient-selection biomarkers that were linked with improved response; notably, age greater than 49 years old was linked with greater improvement in Milner Maze Test (speed p=0.095, accuracy p=0.047) and increase in effect size (Cohen’s d) by 41% and 118% respectively, while baseline systolic blood pressure greater than or equal to 121 mmHg was linked with greater improvement in Milner Maze Test (speed p=0.055, accuracy p=0.052) and increase in effect size by 86% and 157% respectively.
This analysis of Phase 1 data in healthy participants demonstrates the ability of eXplainable AI tools, such as KEM®, to integrate and analyze broad and heterogeneous sources of data from different trials, to provide insight into a drug’s mechanism of action, to generate testable hypotheses, and to guide the optimal design of the next steps in clinical development.
“We are very pleased to report initial results from our collaboration with the Cyclerion team that illustrate the power of integrating our xAI driven approach very early in clinical development. Our first evidence of clinical improvement of endpoints relevant in multiple diseases as well as the systematic identification of candidate patient selection criteria will help the design of next-phase studies with higher probability of success” commented Mohammad Afshar, Ariana Pharma CEO.
References:
-
- – ClinicalTrials.gov Identifiers: NCT03856827, NCT04240158
-
-
- – Poster: CY6463 administration in healthy participants was associated with improvements in Alzheimer’s disease relevant biomarkers based on a systematic analysis of multiple Phase 1 clinical trials using KEM® eXplainable AI.download here
-
-
-
- – Download the Press Release here
-
For further information, please contact: Email: info@arianapharma.com
About Ariana Pharma
Ariana Pharma is a leading Artificial Intelligence (AI) drug development company. Using its KEM® Artificial Intelligence (xAI) technology, Ariana helps its partners introduce personalized medicine clinical trial design into their protocols and optimize clinical endpoints, identify biomarkers of therapeutic response and potential synergistic therapies.
Ariana routinely collects and combines clinical data with omic data, immunological readouts (such as Fluorescence-Activated Cell Sorting (FACS)), microbiota, Patient Reported Outcomes (PRO) as well as Real World Evidence (RWE) data. Combining advanced data analytics, drug development, and regulatory expertise, Ariana helps translate findings into innovative clinical development plans and regulatory approvals.
With a growing number of successful therapeutic development programs, KEM® is an FDA-assessed technology that systematically explores combinations of biomarkers, producing more effective biomarker signatures for precision medicine. Founded in 2003 as a spin-off of the Institut Pasteur, Paris, France, the company operates a subsidiary in the United States since 2012.
Further information is available at www.arianapharma.com
Ariana Pharma Media
Thomas Turcat
t.turcat@arianapharma.com
Ariana Pharma Business Development
Marion Soto, Vice President, Business Development
m.soto@arianapharma.com