Our Business
Ariana’s eXp.AI Driven Drug Development Platform :
Accelerated Path From Design to Approval
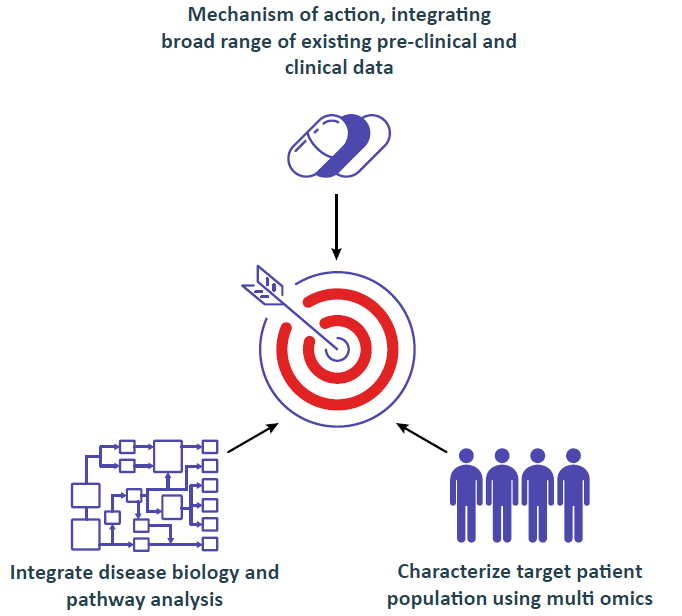
AI Driven Knowledge Integration and Discovery Platform Aiming at Regulatory Strategy

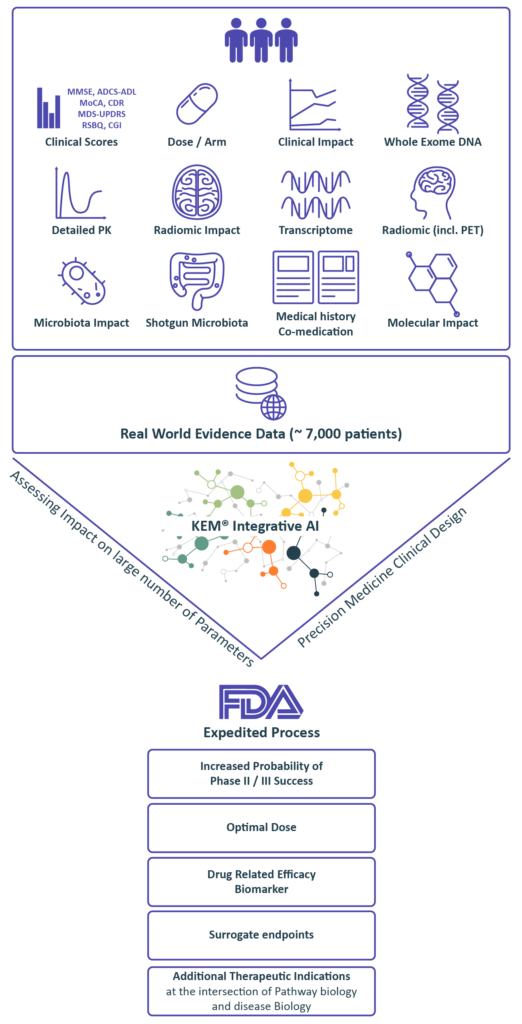
Integrating 100,000s parameters, starting with as few as 10s of patients
Partnerships
RWE Patients Records
Years of experience
Total Investment
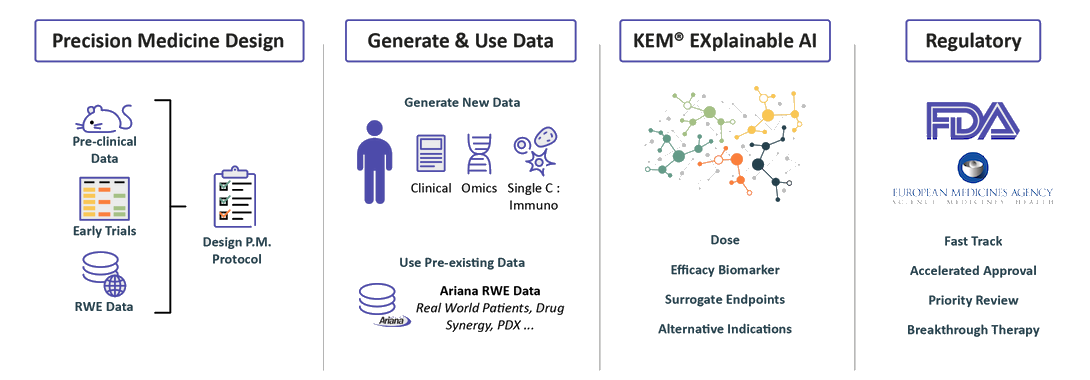
We design Precision Medicine Clinical protocols
We can start our process at the pre-clinical
stage or with as few as 10 patients.
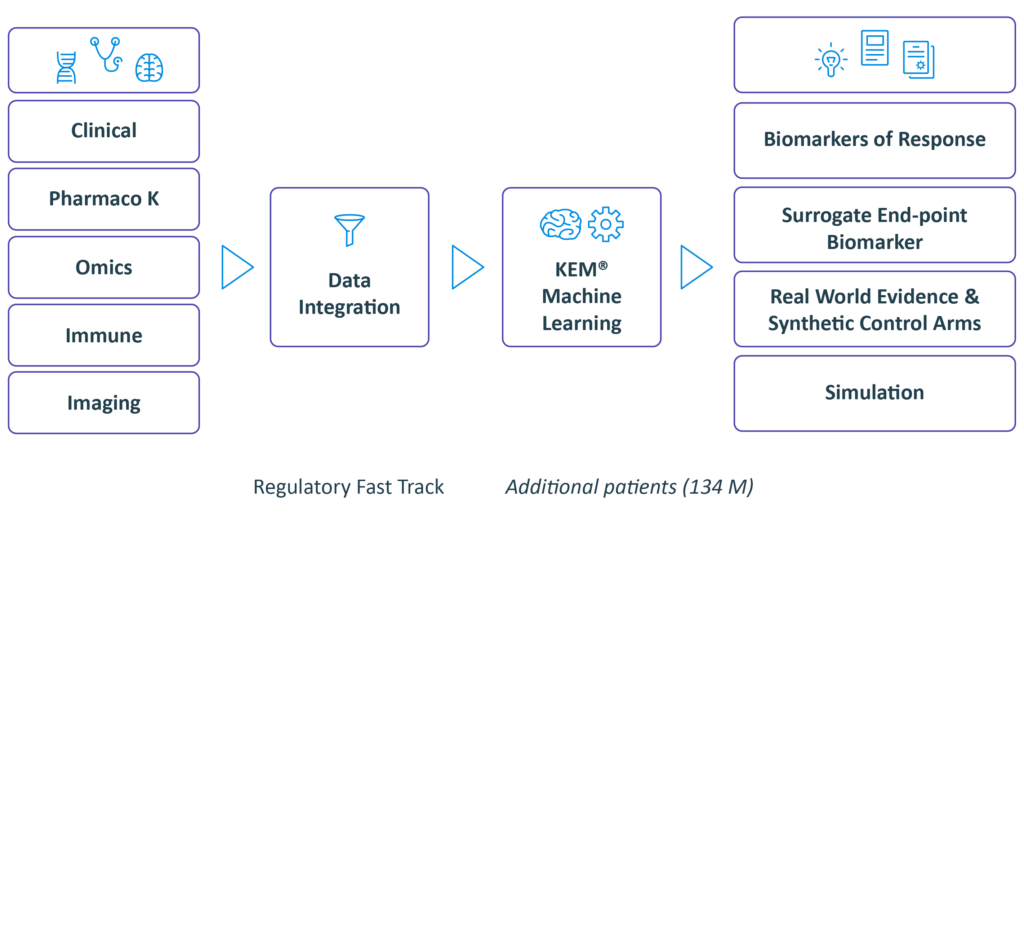
We integrate pre-clinical data, data from
past clinical trials when they exist.
Real World Evidence data from our extensive knowledge base in order to identify biomarkers and design a data enriched clinical protocol.

We collect & generate data

Integrate and discover with our eXp.AI Technology
we identify:
• Optimal dose
• Companion biomarkers and best responders end-points and surrogate endpoints
• Synergistic drug combinations
• Novel indications

Regulatory Strategy
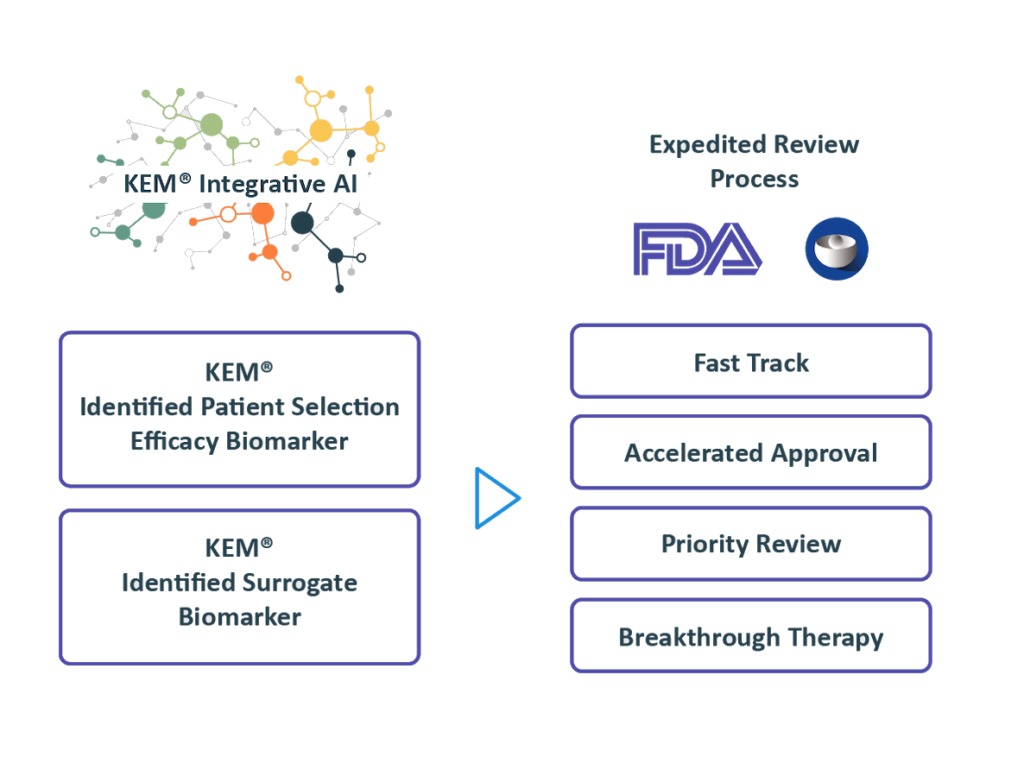

Benefiting from over 10 years of close interactions with the FDA,
we transform findings from the KEM® platform into optimal regulatory strategies for accelerated approval
KEM® Technology validated by a large number of partners


Recent Articles
Latest Press Releases
25Nov
Ariana reaffirms its position on drug development and publishes its new manifesto.
We all share the same challenge: finding the right therapy for the rig...
21Nov
Ariana Pharma enters a new era with Dr. Pascale Bouillé taking the helm of the company.
Pascale Bouillé is the CEO of Ariana Pharma, where she leverages her ...
30Oct
CorpusAnalytiX and Ariana Pharma Announce Collaborative Alliance to Accelerate AI-Driven Drug Development
Collaboration connects CorpusAnalytiX’s diverse healthcare data mark...
