Oct2022
AI -driven analysis of Parkinson’s Disease Dementia clinical trial reveals potential molecular mechanism of blarcamesine (ANAVEX®2-73) in restoring key neurodegenerative pathways
Blarcamesine significantly restores functionality in key molecular pathways including Alzheimer’s and Parkinson’s disease, KEM® xAI analysis shows.
Cambridge, MA, USA and Paris, France, October 4th, 2022 – Ariana Pharma, the leading Al-driven precision medicine company, has announced the first comprehensive blarcamesine pathway analysis impact using extensive data from the ANAVEX®2-73-PDD-001 Parkinson’s Disease Dementia (PDD) clinical study.
Initial results were presented by Ariana Pharma at the Alzheimer’s Association International Conference (AAIC 2022) in San Diego, in collaboration with Anavex Life Sciences Corp. (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of CNS diseases.
Building on extensive multi-omic analysis using Ariana’s KEM® (Knowledge Extraction and Management) AI platform, the findings further establish blarcamesine as a potent modulator of key pathways in multiple neuro-degenerative diseases and provide additional insight into its molecular mechanism of action. Blarcamesine (ANAVEX®2-73) is a novel oral selective sigma-1 receptor (SIGMAR1) agonist. It was previously investigated in a clinical Phase 2a study in Alzheimer’s disease in which blarcamesine resulted in a lower rate of both cognitive and functional decline.
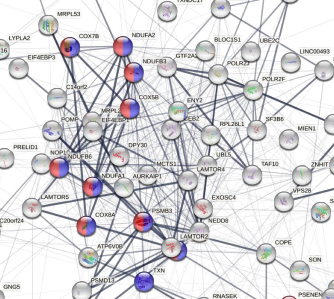

Pathway enrichment analysis revealed that multiple neurodegenerative pathways, including Alzheimer’s disease and Parkinson’s disease were significantly enriched for these genes
Following the positive results of this study, a translational approach led to investigating blarcamesine in an international, double-blind, multicenter, placebo-controlled Phase 2 clinical study of 14-week duration in 132 patients with Parkinson’s disease dementia (PDD). The analysis shows that blarcamesine significantly restores functionality in key pathways including Alzheimer’s disease, Parkinson’s disease. Expression levels of pathological down-regulated neurodegenerative genes of both Alzheimer’s and Parkinson’s were significantly reactivated by the therapeutic effect of ANAVEX®2-73 (p<0.005). Neurodegenerative pathways were identified as significantly overrepresented. Importantly, Down-regulation of genes involved with neurodegeneration was observed in the placebo arm, however, was compensated in the blarcamesine arm.
Whole blood transcriptomics analysis (RNAseq, 14,150 genes) was performed at baseline and end of study (Week 14). The KEM® platform was used to identify clusters of genes that show a correlated expression change across patients and timepoints. In addition, Ariana Pharma identified a novel gene network that is differentially expressed in Parkinson’s disease dementia (PDD) patients treated with ANAVEX®2-73 compared to placebo after 14 weeks of treatment.
Ariana Pharma’s CEO Mohammad Afshar comments: “This first AI-driven pathway analysis in Parkinson’s disease dementia allows us to understand the intersection between a specific drug and patient biology. It further illustrates the power of KEM® Explainable AI to accelerate and increase the probability of success of precision medicine translational drug development through the better understanding of gene pathways in specific indications.”
The identification of a gene network as the blarcamesine response pathway lays the foundation to better understand the mechanism of action at the molecular level of blarcamesine. It is thus unlocking characterization of responders based on molecular profiling, as well as identification of new indications in neurodegenerative and other disorders.
References:
- – Poster: Study of the Mechanism of Action of blarcamesine (ANAVEX®2-73): Whole Blood Transcriptomics Analysis (RNAseq) Identifies Treatment Impact on Compensatory Pathways by Restoring Key Neurodegenerative Pathways Functionality, including Alzheimer’s and Parkinson’s Disease Pathways (ClinicalTrials.gov Identifier: NCT04575259) download here
- – Download the Press Release here
For further information, please contact:
Email: info@arianapharma.com
About Ariana Pharma
Ariana Pharma is a leading Artificial Intelligence (AI) drug development company. Using its KEM® Artificial Intelligence (xAI) technology, Ariana helps its partners introduce personalized medicine clinical trial design into their protocols and optimize clinical endpoints, identify biomarkers of therapeutic response and potential synergistic therapies.
Ariana routinely collects and combines clinical data with omic data, immunological readouts (such as Fluorescence-Activated Cell Sorting (FACS)), microbiota, Patient Reported Outcomes (PRO) as well as Real World Evidence (RWE) data. Combining advanced data analytics, drug development, and regulatory expertise, Ariana helps translate findings into innovative clinical development plans and regulatory approvals. With a growing number of successful therapeutic development programs, KEM® is an FDA-assessed technology that systematically explores combinations of biomarkers, producing more effective biomarker signatures for precision medicine.
Ariana has developed Onco KEM®, the most advanced, clinically tested, oncology therapeutic decision support system. Founded in 2003 as a spin-off of the Institut Pasteur, Paris, France, the company operates a subsidiary in the United States since 2012.
Further information is available at www.arianapharma.com
