Transcriptomics in Action: Pioneering Personalized Cancer Treatment through the WINTHER Trial
Overview
The WINTHER clinical trial represents a pioneering endeavor in personalized cancer treatment through the use of transcriptomics. This trial, led by Professor Jean-Charles Soria and involving multiple international institutes (MD Anderson Cancer Center, University of California San Diego Moores Cancer Center, Vall d’Hebron Institute of Oncology, Chaim Sheba Medical Center, Ben-Gurion University of the Negev, Centre Léon Bérard, Segal Cancer Centre, McGill University), applied gene expression analysis to guide therapy choices for patients with various cancers, particularly when DNA alterations did not offer clear treatment pathways.
Impact
• WINTHER’s approach, utilizing dual biopsy transcriptomic analysis compared to matched normal tissue, broadened the applicability of precision medicine. By incorporating RNA data, the trial demonstrated that patients without actionable DNA targets could still receive personalized therapies based on RNA expressions.
• The trial contributed to advancing cancer treatment protocols by demonstrating the utility of RNA data in enhancing treatment matching and improving patient outcomes, thus setting a new standard in the field of oncogenomics.
Objectives
• To expand the application of precision medicine by integrating transcriptomic data into clinical decision-making, offering therapeutic options for patients who lack identifiable DNA targets.
• To develop and validate a novel algorithm that uses RNA information from patients to link with an extensive knowledge database, providing personalized therapy recommendations.
Method
WINTHER has compared the outcome of patients treated in the first arm by a therapy chosen based on a DNA profiling of the tumor from Foundation Medicine, to the outcome of patient in the second arm where therapy was chosen using a unique and innovating methodology combining differential gene expression analysis between tumor tissues and matched healthy tissues and our AI algorithm Onco KEM®, matching tumor transcriptomic profile against a comprehensive drug database enabling the identification of optimal treatment regimens based on RNA findings.
Results
• The trial achieved significant success in increasing both Overall survival and the matching rate of patients to effective therapies through RNA analysis, evidenced by a notable case where a patient with gastrointestinal neuroendocrine tumors showed prolonged disease stabilization under treatment guided solely by transcriptomic data.
• This method demonstrated a considerable improvement in the precision of cancer treatment, particularly for patients who traditionally would not benefit from genomics-guided therapies.
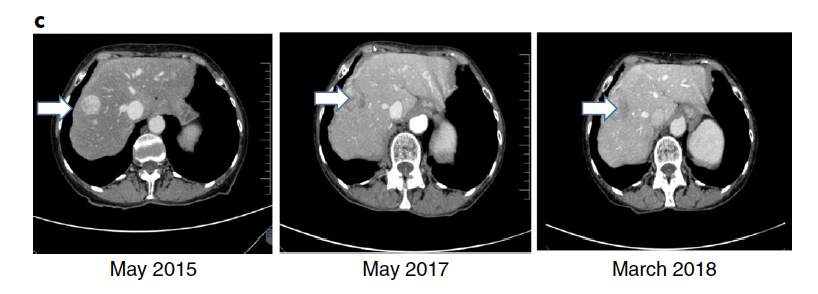
Examples of exceptional responses with CT scans: A 69-year-old woman with a well-differentiated, neuroendocrine, small gut tumor and peritoneal metastasis underwent debulking surgery and received lanreotide (a long-acting somatostatin analog) for residual disease (April 2011 to June 2012). She then developed bowel obstruction (due to peritoneal progression), which required surgery. The somatostatin analog continued until April 2014, when progression to the liver occurred. Axitinib (on a clinical trial) was given for 10 months before progression. In April 2015, she enrolled on the WINTHER trial. NGS found no DNA alterations; RNA matches revealed AKT2 and AKT3 overexpression. The WINTHER CMC recommended an mTOR inhibitor. Everolimus was started (May 2015). Outcome refers to prolonged disease stabilization (PFS of >34 months). (Everolimus was later approved for this indication in 2016.) The left panel shows baseline liver metastases (arrow). The middle panel shows stable liver metastases (arrow) at 1 yr. The right panel shows ongoing stable liver metastases (arrow) at >34 month