Imcyse:Revolutionizing diabetes care with only 41 patients
Overview
New type I diabetes treatment, IMCY-0098, a proinsulin-derived Imotope™ in Phase I developed by Imcyse with first results in only 41 patients
Impact
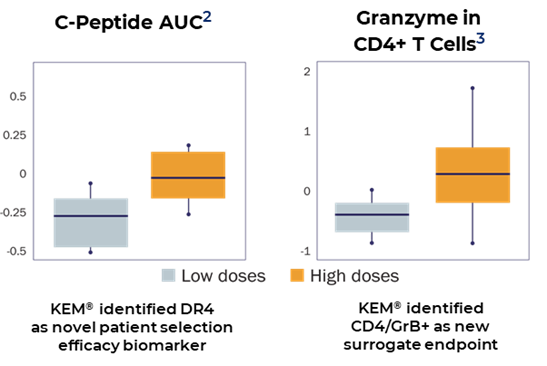
• Hypotheses of biomarkers identified by KEM® were implemented and confirmed in phase II with positive results.
• Positive Phase 1b data triggered Series B Pre-IPO funding of $40m
KEM®’s unique abilities in the unbiased analysis of small datasets with eXplainable AI enabled Ariana to deliver actionable and powerful insights on IMCY-0098 therapy.
Objectives
• Identify patient profiles with the highest probability of response to IMCY-0098 therapy.
• Identify surrogate biomarkers, linked with the therapeutic impact of the treatment, acting as accurate substitute of clinical endpoints.


Method
KEM® systematically associated the treatment and baseline characteristics with the efficacy of the treatment as measured by insulin intake, autoantibodies levels, MMTT (Mixed Meal Tolerance Test), and glycaemia. KEM® enabled the systematic exploration and ranking of all connections between treatment and markers. The explainability of KEM® provided a support to investigators to make a decision for further clinical development plans.
Results
KEM® identified haplotype HLA DR4 as the most responsive subgroup. And CD4/GRB+(CD4, granzyme B+ T-cell) was associated with the dose and identified as a surrogate endpoint.