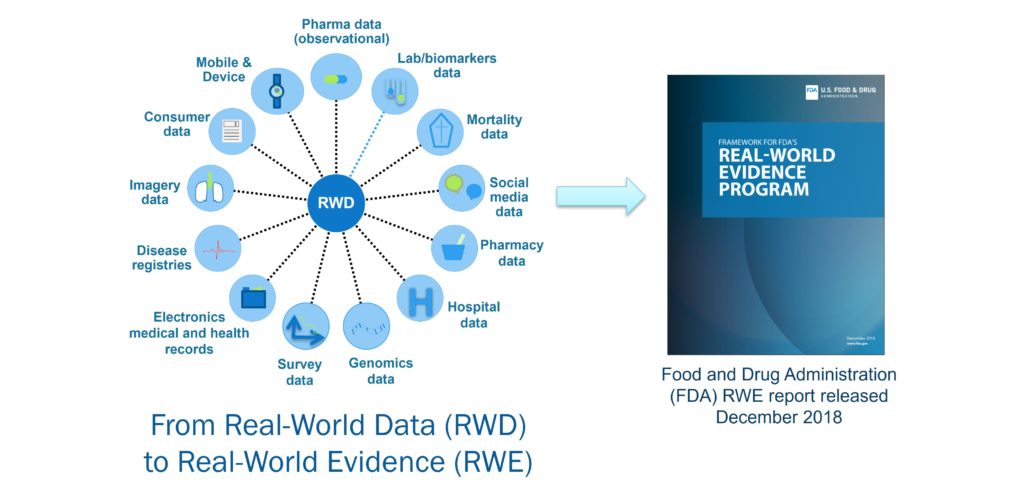
Real World Evidence
Ariana® Real World Data & Evidence (RWD/RWE) platform capabilities
- • Early Detection of Genetic Risk Factors
- • Personalized Preventive Strategies
- • Optimized Vaccination & Drug Response
- • Reducing the Burden of Chronic Diseases
- • Enhancing Public Health Policies
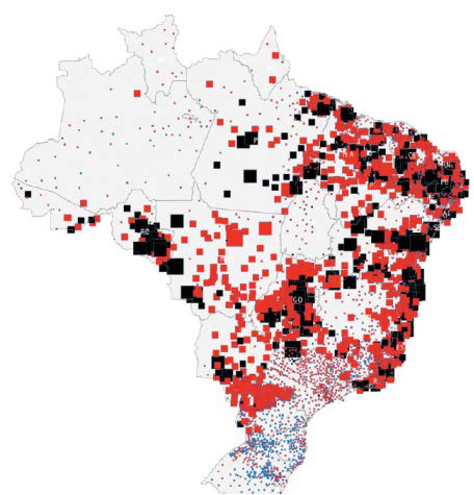

Global Health Assesment
- Ariana has developed a sophisticated and customizable cloud-based analytics and reporting platform, designed to generate robust evidence from Real-World databases, with applications in domains such as:
- ● Epidemiological studies
- Epidemiological studies of the medico-economic impact of specific diseases or industrial and environmental activities. Analyzing healthcare costs, economic burdens, and public health outcomes to guide policy decisions and resource allocation.
- ● Early Detection of Genetic Risk Factors
- By analyzing the genetic makeup of a population, identifying predispositions to hereditary diseases such as cardiovascular disorders, diabetes, and cancer. Individuals at higher risk can receive personalized screening plans and early interventions to prevent disease onset.
- ● Personalized Preventive Strategies
- Designing preventive medicine strategies to tailor to individual’s genetic risk profile, Instead of using a one-size-fits-all approach. For example, those with a genetic predisposition to hypertension may receive targeted lifestyle and dietary recommendations before symptoms appear.
- ● Optimized Vaccination & Drug Response
- Base on identified genomic profiling, predict how individuals will respond to vaccines and medications. Enabling healthcare providers to adjust dosages or select alternative treatments to maximize effectiveness and minimize adverse reactions.
- ● Reducing the Burden of Chronic Diseases
- Many chronic diseases, such as diabetes, are influenced by genetics. Early genetic screening, enables healthcare providers to proactively manage risk factors (e.g., through dietary modifications, physical activity, or medical supervision), ultimately reducing the prevalence of these diseases
- ● Enhancing Public Health Policies
- With a deeper understanding of genetic risk factors at a population level, enabling the implementation of targeted public health programs, such as genetic counseling initiatives or specific health campaigns. Policies can be adapted to high-risk groups, ensuring that healthcare resources are allocated efficiently.
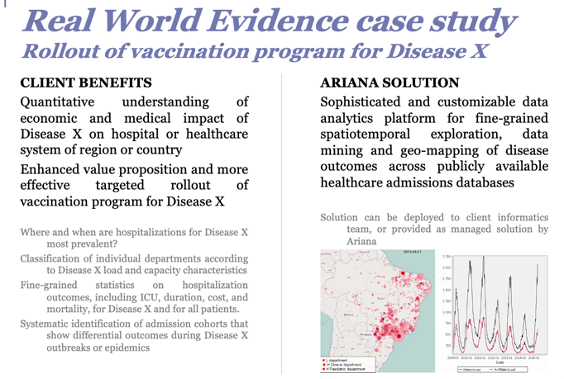

Synthetic control arm for clinical development

High quality curated electronic health records (EHR) data collected during routine clinical care of patients have the potential to accelerate drug development
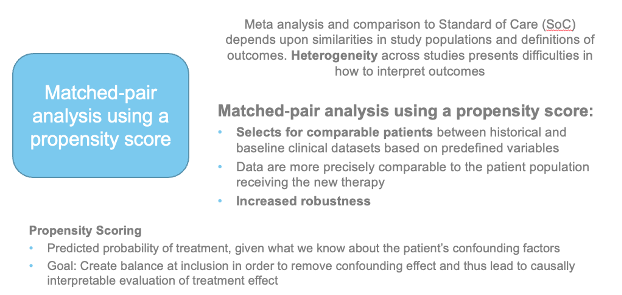
In areas of high unmet need, real world controls could help provide external control for regulatory decision-making
Requires advanced analytical techniques to address limitations pertaining to RW data (propensity scores)
« Real world patients » may be different to patients enrolled in clinical trials (with comorbidities, etc.) and use of RW data may provide a more accurate picture of the impact of a novel therapy.

Scientific Publications
Augmenting single-arm Phase 2a open label trials with Real-World external control data.
Afshar et al. 2019 J Prev Alzheimers Dis 6, 45–154.
Mortality among hospitalized dengue patients with comorbidities in Mexico, Brazil.
Macias et al. 2021 AJTMH, 105(1), 102–109.
Real-World Evidence of Dengue Burden on Hospitals in Mexico: insights from the automated...
Macias et al. 2019 RDI Clinica, 71(3), 168–177.
Comorbidities increase in-hospital mortality in dengue patients in Brazil.
Werneck, et al. 2018 MDI Oswaldo Cruz, 113(8), 1–5.
