Advancing Clinical Trials: The Role of Synthetic Control Arm
Overview
Ariana successfully created a Synthetic Control Arm (SCA) to evaluate the efficacy of a treatment against Alzheimer. Leveraging its Real World Data (RWD) capabilities, Ariana applied matching strategy to identify untreated patient from a public database having the same clinical characteristics and inclusion criteria of treated patients from an Alzheimer clinical study. SCAs offer a desirable alternative to traditional randomized controlled trials (RCTs) by allowing for the comparison of treated patients against a cohort of untreated patients digitally created.
Impact
• SCAs address ethical concerns associated with withholding potential treatments from control groups in life-threatening conditions or when no standard treatment exists. They also mitigate challenges in recruiting sufficient patients for rare or progressive diseases.
• Within the KEM® platform, the SCA module designed by Ariana provides a sound basis for assessing treatment efficacy, potentially accelerating drug development and approval processes.
Objectives
• Leverage comprehensive RWD to construct SCAs that accurately reflect the characteristics of traditional trial control arms without the enrollment of actual patients into placebo groups.
• Provide an assessment of treatment efficacy.
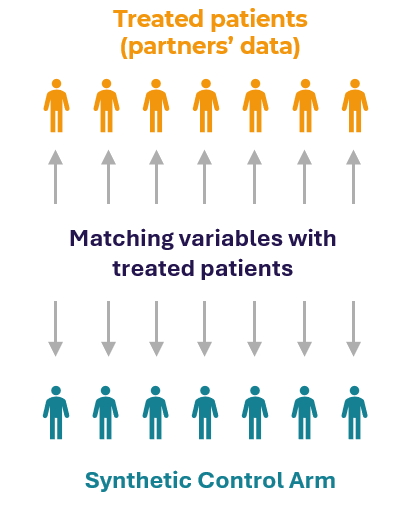

Method
Matched controls were selected from extensive external databases using propensity score combined with advanced matching algorithms (e.g., nearest neighbor matching, genetic matching) that consider multiple baseline characteristics such as age, sex, and disease severity. These synthetic arms are then used to mimic control groups in clinical trials, providing a comparator for treated patients.
Used Case
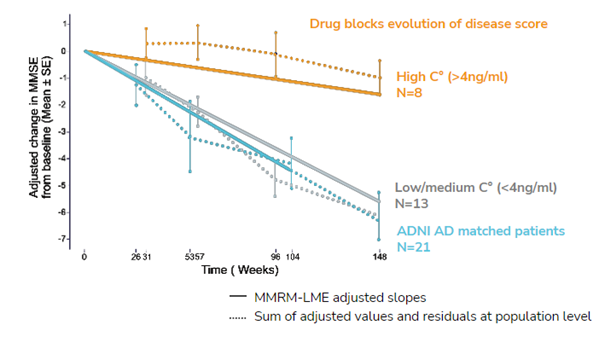
The SCA module of Ariana combined with Real World Data (RWD) capabilities were used to assess the efficacy of a phase IIa Alzheimer treatment. The synthetic control arm was constructed from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database.It demonstrated significant efficacy in mirroring the natural progression of untreated Alzheimer’s disease, thereby validating the therapeutic effects of the drug treatment.