Rare disease: Identification of new biomarker with 31 patients
Overview
A randomized Phase II study involving 31 patients with Rett Syndrome.
Participants were enrolled in a 7-week study to assess the safety, tolerability, and efficacy of ANAVEX®2-73 treatment.
Impact
- ● The identification of a new biomarker and its follow-up in the study enabled a Fast Track designation granted by the FDA
- ● Successfully randomized Phase II results
- ● Surrogate biomarker available for Phase 3 study.
Objectives
- Identify a new biomarker to define a group of patients who respond better to the treatment.
- Identify a surrogate biomarker of response to measure the treatment’s impact at the biological level.
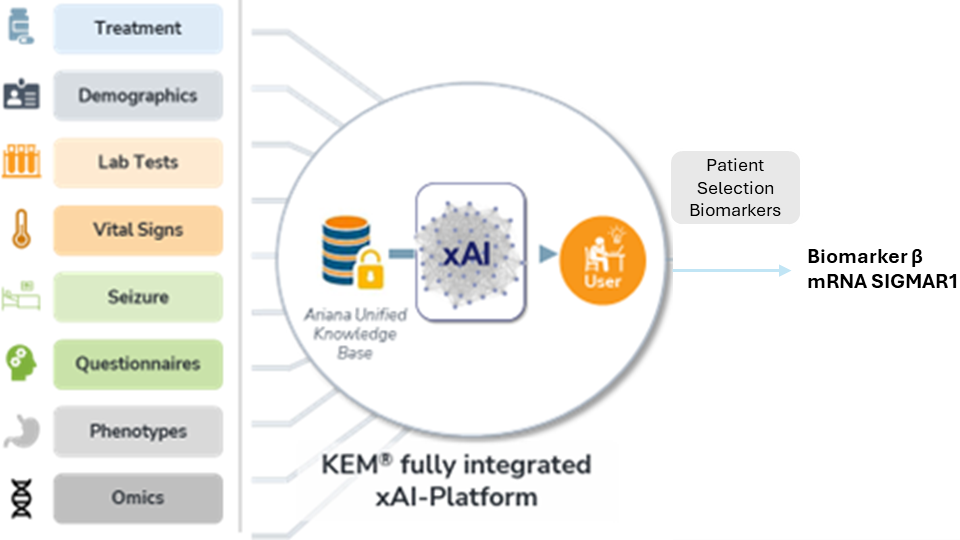

Method
Ariana had identified a specific variant of SIGMAR1 as a candidate biomarker of response for ANAVEX®2-73 during a study on Alzheimer’s patients. In this context, the treatment or a placebo was randomly administrated to 29 new patients with Rett Syndrome during an additional clinical trial.
KEM® was applied to 8,000 variables, including cognitive tests,Ra omics, and phenotypic data.
Result
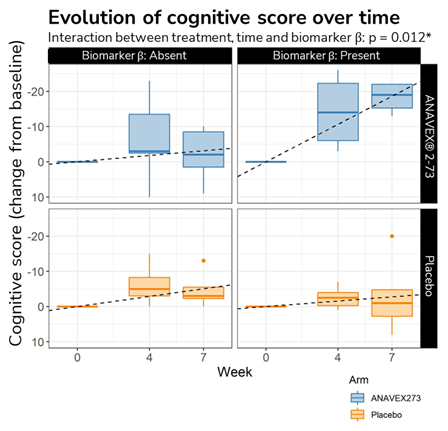
● Identification of a new candidate biomarker:- – Cognitive Scores significantly improving for patients with a high value of one of the psychometric score evaluated at baseline.
- – A longitudinal study confirms the positive interaction across time between the treatment and a high value of one of the psychometric score evaluated at baseline.
● On the top of this, the mRNA expression of SIGMAR1 was identified as surrogate biomarker of response.