Identifying best responder biomarker with only 45 patients in new imuno modulatory treatment in combination with anti-PD-(L)1 therapy
Overview
Phase I/II study on patients with solid cancer. New imuno modulatory treatment in combination with anti-PD-(L)1 therapy.Impact
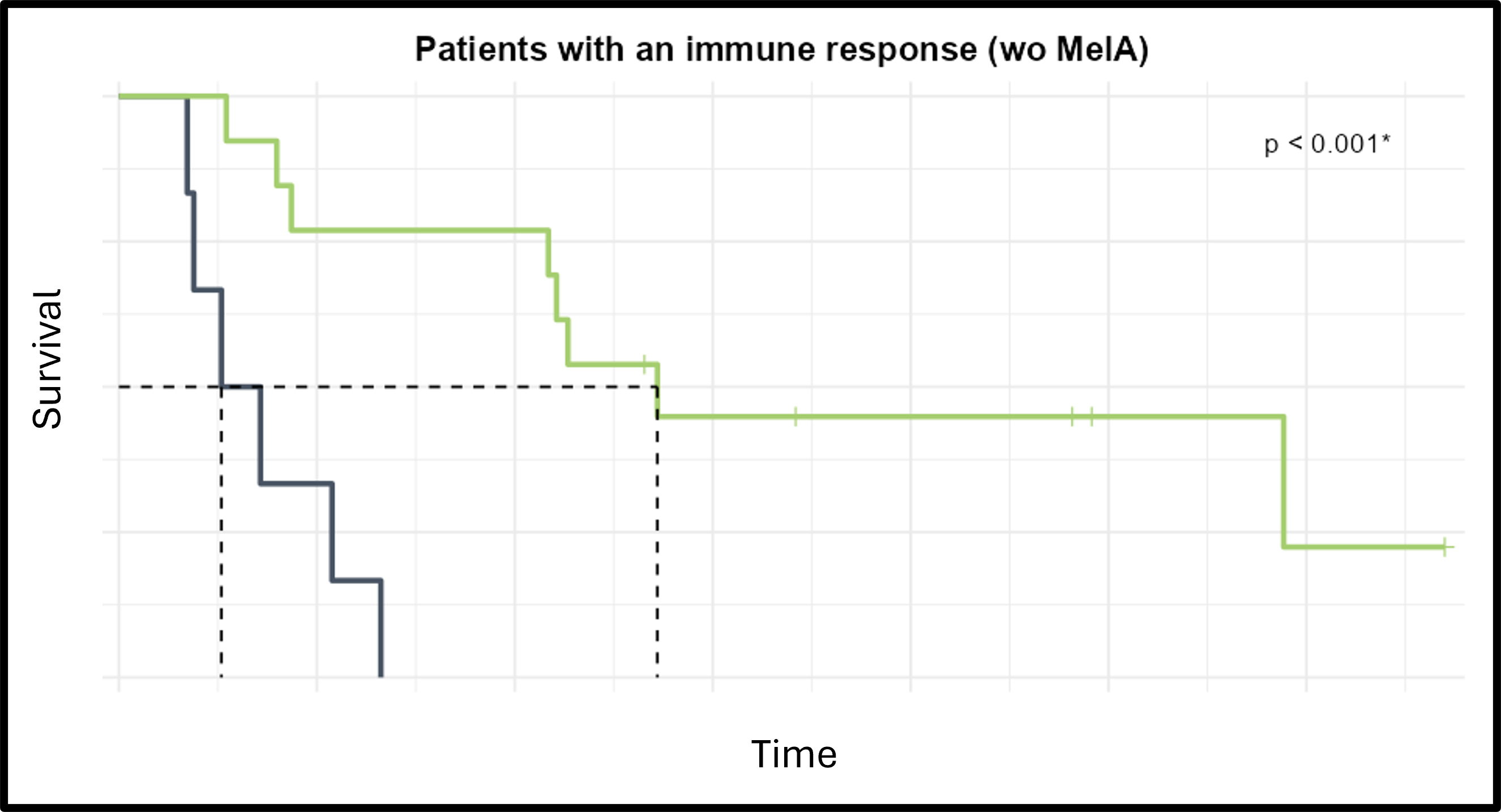
- ● A subgroup of responding patients was identified, paving the path to a precision-medicine design for the following phases of the trial
- ● Within the observed subgroup, the PFS is compared to the other patients with an immune response
- ● The immune response to the combined therapy was characterized, helping a better understanding of the mechanism of action
Objectives
Identify baseline biomarker increases the chances to transform the immune response into a clinical improvement i.e. higher survival.

Method
Heterogeneous data from 45 patients were integrated into a consolidated database with 404 variables. All patients received the vaccine in combination with an anti-PD-(L)1 therapy.
KEM® explored all associations between the variables in order to identify subgroups of patients with higher chances of response to the combined therapy, both at the immune and clinical level.
Results
Kem ® identified three groups of patients:- ● Patients with biomarker 1 at baseline that have both an immune and a clinical response.
- ● Patients with biomarker 2 at baseline that show an immune response but no clinical improvement.
- ● Patients with biomarker 3 at baseline that show clinical improvement despite the absence of immune response. The clinical improvement may be driven by the combination of vaccine with anti-PD-(L)1 therapy.