eXp.AI-driven dose justification and biomarker identification
Overview
Phase II study on patients with Cutaneous T-cell Lymphoma. The new drug in development is a KIR antibody with fixed dose.Impact
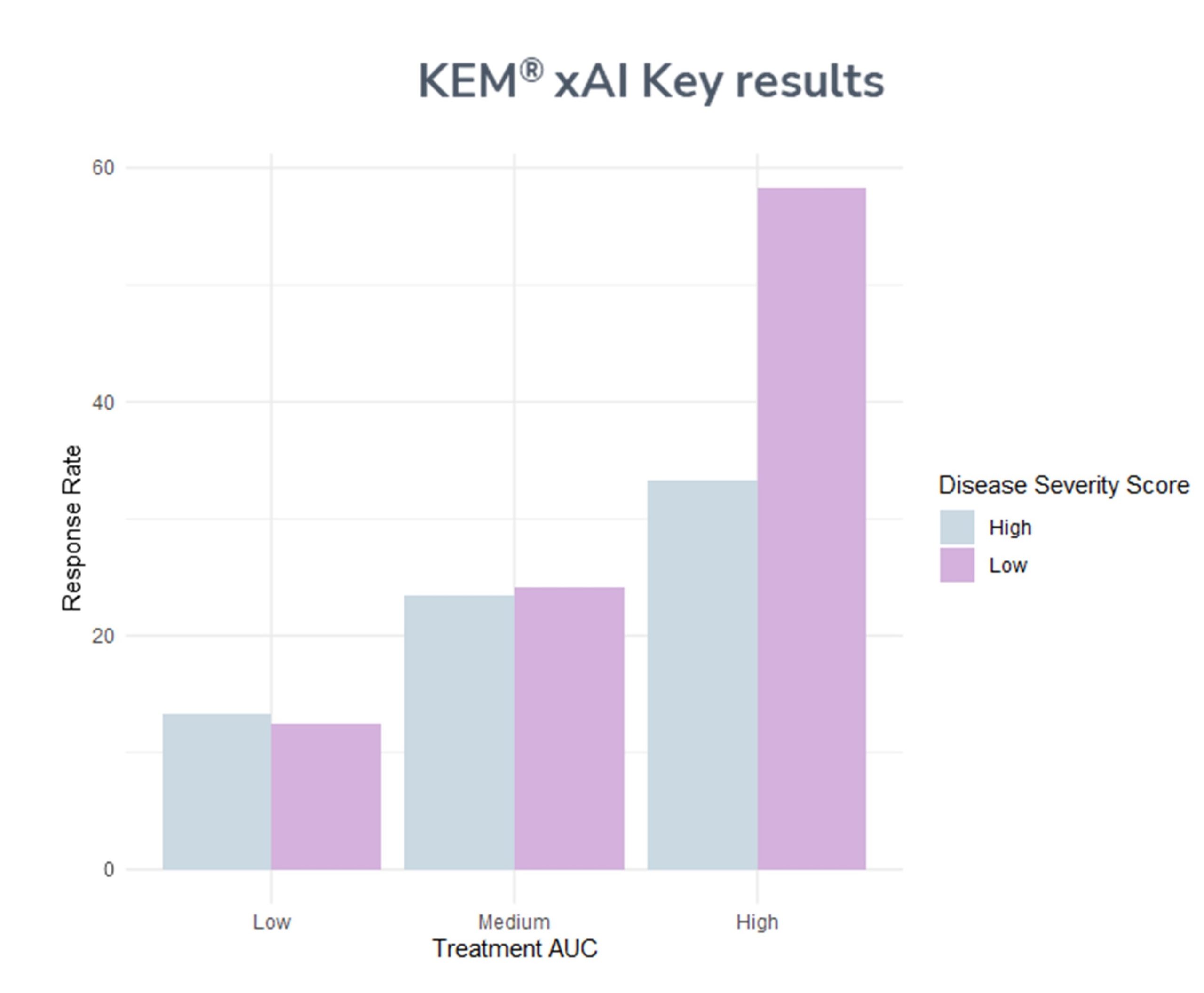
- ● Higher Exposure (Area Under Curve, AUC) is positively linked with response, suggesting lowering treatment dose would decrease efficacy
- ● Among high AUC patients, a subgroup of best responders was identified, creating new design opportunities for the following trial phases
- ● Within the observed subgroup, the response rate is 1.75 times higher
Objectives
The objective is to provide robust justification for the selected dose, and to establish whether this dose is safe and can trigger a response in Cutaneous T cell Lymphoma patients.

Method
To assess the efficacy of the treatment, we used the KEM® eXp.AI platform to identify all relationships between treatment exposure and response to the treatment in a hypothesis-driven manner, using pertinent categories of variables impacting response to drug, such as patient age, sex, weight and disease severity.
To obtain a more exhaustive view of the variables that could impact patient response to treatment, KEM® explored all relationships between treatment, patient characteristics and response to treatment.
Most pertinent relationships in term of metrics and clinical interpretation were then extracted and retained.
The safety of drug was evaluated in a similar manner by exploring the relationships between exposure to treatment and safety events.
Result
- ● The selected dose is the appropriate dose needed for therapeutic response in the patient population.
- ● High exposure to drug is not associated with increased risk of serious adverse events.