Breaking New Ground in Alzheimer's Care with only 32 patients cohort
Overview
Recent findings in Alzheimer’s disease (AD) have identified the Sigma-1 receptor as a possible therapeutic target: ANAVEX Life Sciences Corp is developing ANAVEX®2-73 (BLARCAMESINE), a Sigma-1 receptor agonist as new treatment for AD. Leveraging the throughput of Next Generation Sequencing for biomarker identification, Ariana helped ANAVEX Life Sciences Corp move forward along its clinical development path, advancing from initial Phase I-II studies to Phase III, with the first results revealing substantial promise in precision medicine based on a genome-wide search for biomarkers, beginning with a small cohort of 32 patients.
Impact
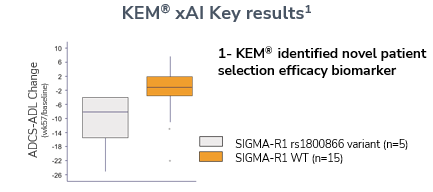
• KEM® analytics aided in discerning novel biomarkers for patient efficacy, tailoring treatment to those most likely to benefit.
• The elucidation of these biomarkers has led to three new potential indications, Rett Syndrome, Dementia and Parkinson’s Disease, with significant regulatory strides such as Fast Track designation.
• Financially, this research has been transformative, with ANAVEX’s market valuation experiencing a monumental rise from $150M to over $1B, post-study.
Objectives
• To identify key biomarkers that predict therapeutic response and are validated in longitudinal studies.
• To determine optimal endpoints and pharmacodynamic markers of drug efficacy.
• To expand the utility of ANAVEX®2-73 across multiple neurological diseases by leveraging biomarker-driven precision medicine.

Method
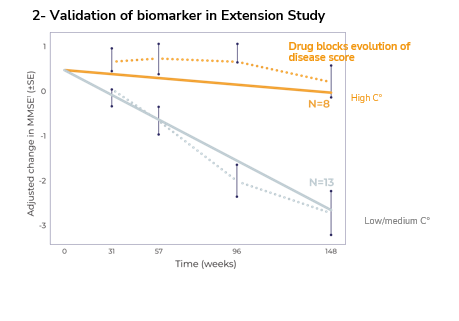
The project harnessed the power of KEM® eXp.AI to analyze complex heterogenous molecular data sets, including genetic and microbiota data as well as real-world evidence data. This approach allowed identification of biomarkers in a Phase IIa that were confirmed in an open label extension study.
Results
• ANAVEX®2-73 demonstrated a statistically significant reduction in disease progression over a 148-week trial period.
• The high-concentration cohort of patients showed an 88% improvement over the low-concentration cohort, maintaining performance in activities of daily living.
• Further genomic analysis solidified the role of known variant with SIGMAR1 as significant key in treatment efficacy.